
Published on June 1, 2023
Reading time: -- minutes
Last updated on December 31, 2024



Applying continued process verification (CPV) plans to continuous production can be challenging due to the large amounts of data it generates and the complex nature of continuous manufacturing. In this blog post, we look at how implementing digital CPV plans can contribute to continuous processes reaping the benefits of Industry 4.0 and digitalization.
For more than 50 years, manufacturers have been producing pharmaceuticals using a method known as “batch manufacturing,” a multistep, lengthy process that involves the use of large-scale equipment. However, advances in manufacturing technology have prompted the pharmaceutical industry to consider moving away from batch manufacturing to a faster, more efficient process known as continuous manufacturing. 1
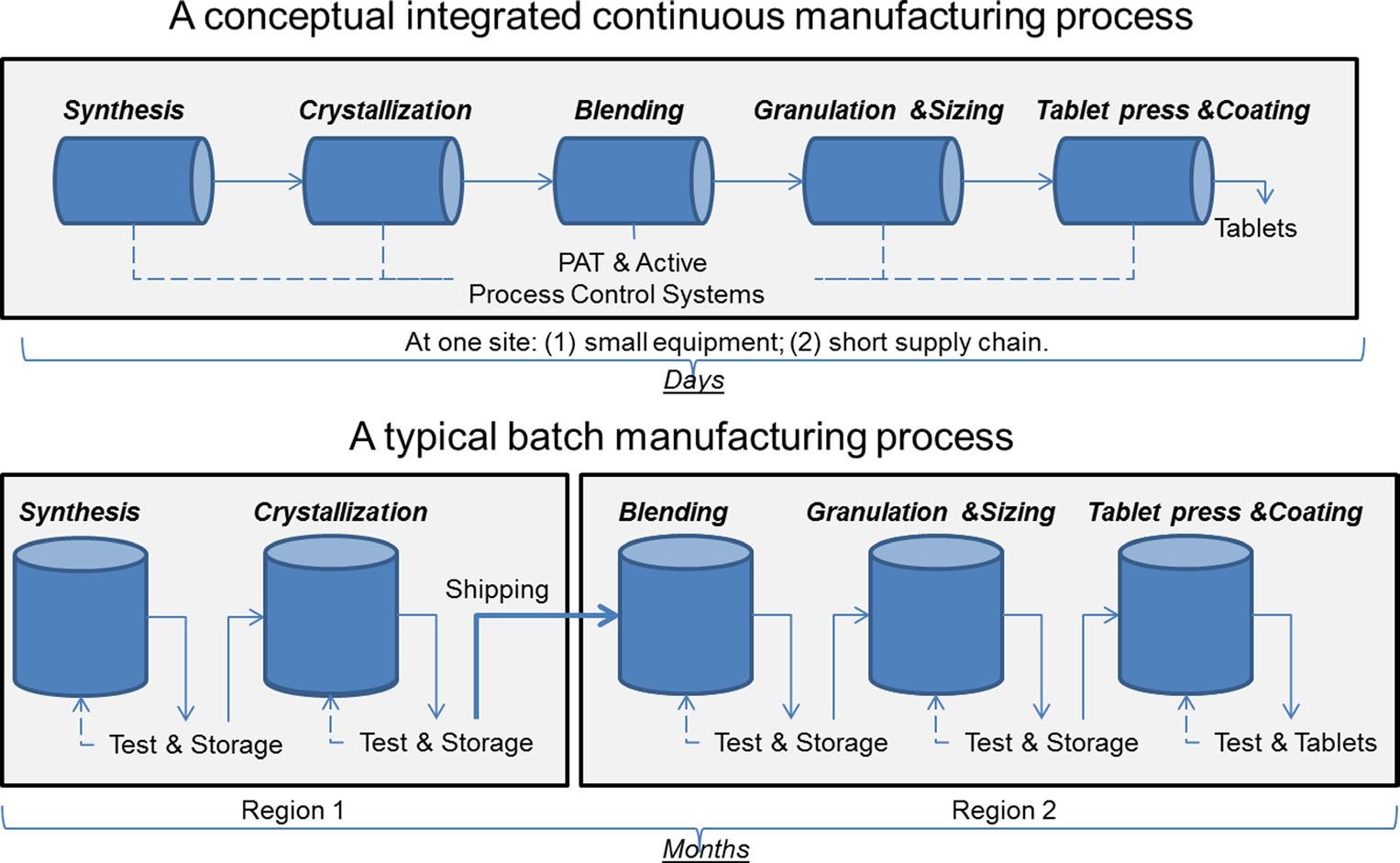
Figure 1: A comparison of continuous manufacturing and batch manufacturing. Image courtesy of Sau Lee. J. Pharm Innov (2015) 10:191-199.
Pharmaceuticals made using continuous manufacturing are continuously transformed within the same facility, eliminating hold times between steps. When compared with traditional batch and fed-batch processes, continuous manufacturing has the following advantages: 2,3
However, these advantages come at a cost: continuous processes have a more complex operation and need in-depth process understanding to achieve and maintain steady-state conditions. Additionally, the initial investment required is high. Naturally, since both types of manufacturing are subjected to the same quality control standards, and the monitoring of continuous manufacturing facilities needs to be more frequent, it should be automated.
This brings us to digital CPV.
A digital CPV program supports the increase in process knowledge and guarantees manufacturing robustness in line with the Quality by Design (QbD) framework.
However, manual CPV programs require a heavy workload to collect and organize the data, build efficient metrics and plots, and update them with new data. The process of generating reports is also labor intensive, and most data extraction, analysis, and collation tasks are repetitive. Manual CPV is also costly because it requires the expertise of highly skilled workers.
In any scenario, whether continuous or batch manufacturing, there are significant differences between manual and digital CPV, as outlined in the table below.
| Risks of a Manual CPV | Benefits of a Digital CPV Plan |
| Data integrity is not assured due to lower data quality resulting from human error in data collection and aggregation. | Assures data integrity and real-time updates by automatically integrating data sources across development phases, securing data traceability and process changes. |
| Dependent on experienced operators to update the data and monitor the CPV plan. |
Reduces the necessary personnel assigned to program, update, accelerate, and automatize report creation with the periodicity defined. |
|
Higher effort placed on data aggregation, organization, and compilation rather than in data analysis. |
Provides a robust and scalable workflow to simplify the implementation of a CPV plan for new products. |
| Evaluates the real-time state of the process by presenting routine monitoring dashboards, allowing quick identification of program improvements. |
Overall, a manual CPV plan provides a reactive approach to problems that may arise, and opportunities for improvement may take longer to be identified. A digital CPV plan allows us to focus on process improvements and proactive behavior.
The steady-state stage of the continuous manufacturing process requires a robust control strategy where the speed at which measurements are acquired is critical to detect process performance and quality deviations proactively. And to complicate things, the continuous nature of the process means there is no possibility to segregate a batch or a set of batches and assess the impact on its quality.
Therefore, a digital CPV plan is a great match for continuous manufacturing processes.
Digital CPV allows for the control of the continuous process by continuously analyzing process data in a multivariate way, making it possible to perform early detection of process faults and define adequate corrective measures in a timely fashion. This, in turn, prevents the process from reaching “points of no return,” a state where the quality impact assessment is complex and will potentially impact product release timelines to supply the market.
Finally, modeling strategies can be used to increase process understanding, comprehend the complex correlations between process parameters and the product outcome, and develop adequate control models.
Due to the many parameters to control, using artificial intelligence or machine learning algorithms such as artificial neural networks, support vector machines, or random forests can increase the control accuracy.
Discover how ValGenesis is transforming the implementation of digital CPV plans. Contact us, and our experts will help you find the solution tailored to your needs.
Sources:
(1) Lee, Sau (Larry). US Food and Drug Administration Center for Drug Evaluation and Research. Current as of 5/17/2017. Modernizing the Way Drugs Are Made: A Transition to Continuous Manufacturing. fda.gov Accessed 5/26/2023.
(2) Gutiérrez-Granados, S., Gòdia, F., & Cervera, L. (2018). Continuous manufacturing of viral particles. Current opinion in chemical engineering, 22, 107-114.
(3) Wahlich, J. (2021). Review: Continuous Manufacturing of Small Molecule Solid Oral Dosage Forms. Pharmaceutics 2021, 13, 1311.

Discover how digital CPV enables real-time quality monitoring in pharma and supports continued process validation across the product lifecycle.
By Maria Batalha
Read
Explore how digital transformation in pharma manufacturing enables continuous process verification and data-driven quality insights for better APQRs.
By Rui Almeida
Read
Discover how digital continued process verification (CPV) drives process optimization in pharmaceutical manufacturing, reducing time, cost, and errors.
By Inês Victorino
Read