
Published on April 20, 2023
Reading time: -- minutes
Last updated on November 4, 2024



Today we want to take a fresh look into comparability for post-approval changes. How? We’re thinking of a unique multiparametric approach. This approach is aligned with the FDA’s tiered and totality-of-evidence concepts in biologic development.
These concepts are helpful to compare biosimilar and reference products. But they can go beyond that! You can use them to perform comparability assessments during the lifecycle management of a given biologic product.
When you’re in the business of producing biological medicines, you will experience some variability over consecutive batches.
For example, the glycosylation profile of a protein. It can present minor differences in its sugar chains without changing its biological activity:

But let’s zoom-in on post-approval changes!
You should expect process post-approval changes as part of your product lifecycle. They happen whenever your site changes, when you scale-up operations, when you replace equipment or when you introduce new analytical methods.
All of this can impact product quality and safety. Because of that, the differences shown by the comparability assessments before and after change should be small. Especially in comparison with products made by two different processes and manufacturers.
We propose that you should use a Multivariate approach. Why should you? Because you need to be able to find very small changes across multiple attributes. And then you need to translate them into an overall assessment of the product changes.
You can implement this approach by comparing analytical packages from pre- and post-change. You do this comparison by applying a comprehensive set of analytical techniques to multiple quality attributes within each package.
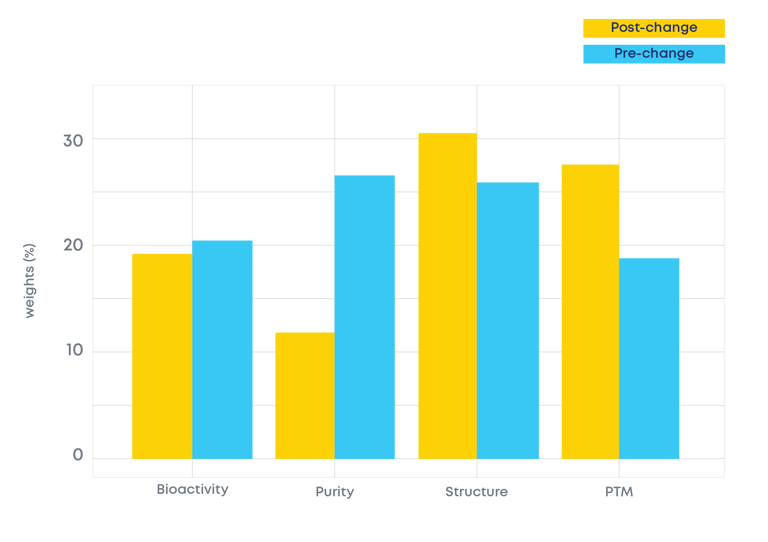
Fig. 2 - Use of multiblock approach to identify the analytical domain representing the major source of variability between the pre-change and post-change product.
Using this approach ensures that the most relevant information on each analytical technique used is captured. All the techniques are then consolidated into a single overall analytical package evaluation. And then you can compare it!
We took a deeper look at this topic in an article that we have just published. You can obtain it from here: Article: Comparability Studies for Post-Approval Changes
The best way to make sure your Tech Transfer yields the best results is to work with specialized consultants.
ValGenesis has a long and established expertise supporting world-class pharmaceutical companies with their Tech Transfers. We do this by using a Quality by Design (QbD) approach and Data-based Process Design decisions.
Take a look at our Technology Transfer Support!

In this blog post, we tell what the most effective approach for your biosimilars approval to reduce its time to market is.
By Catarina Leitão
Read
In this blog post, we will explore how making your transition from the traditional CSV to the new CSA approach can save you time and resources.
By Pedro Ferreira
Read
Learn how to simplify regulatory approval for post-approval changes and discover the benefits of implementing a PACM protocol and using a QRM platform.
By Rui Almeida
Read