How to Apply a Quality by Design Framework with ValGenesis iCMC

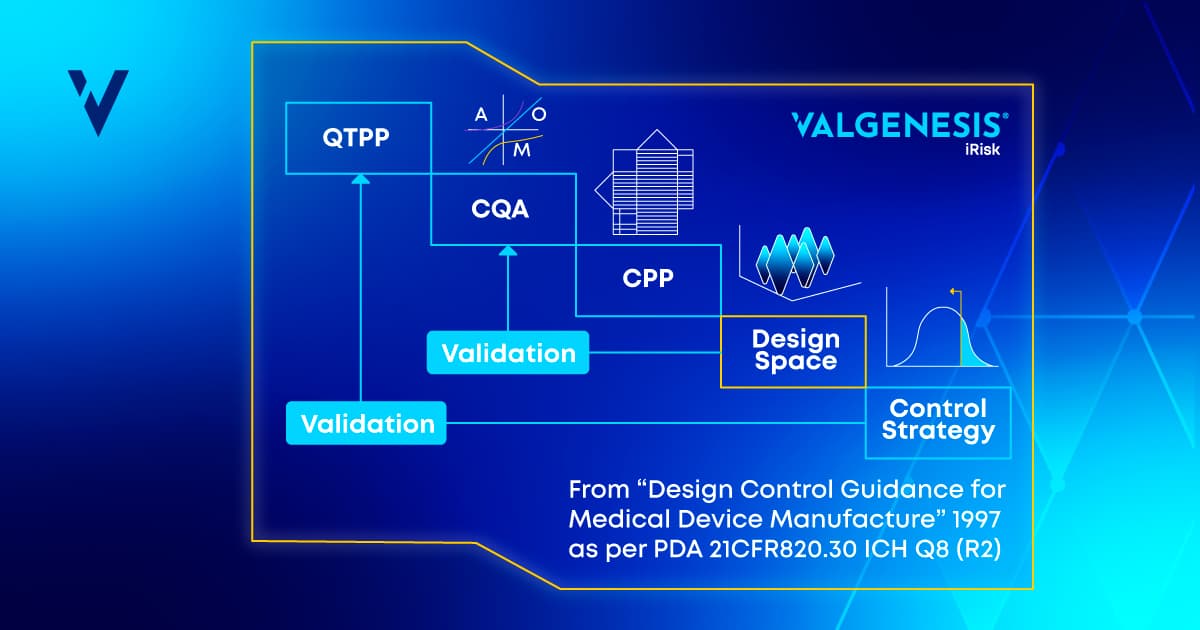
Quality by Design (QbD) is a cornerstone of modern pharmaceutical process development and chemistry, manufacturing, and controls (CMC) data management. As a framework, QbD emphasizes a systematic and proactive approach to ensuring that pharmaceutical products meet predefined quality criteria.
The QbD framework is also endorsed by the International Council for Harmonization (ICH) through guidelines such as ICH Q8(R2) and ICH Q9(R1), which work in concert with scientific guidelines like ICH Q2(R2) validation of analytical procedures to guide method development and validation.
Central to QbD is the identification of critical quality attributes (CQAs) and the establishment of control strategies to manage these attributes throughout the manufacturing process.
In this blog post, we’ll explore how ValGenesis iCMC™ is the ideal tool for facilitating the application of QbD frameworks in CMC lifecycle management, providing a robust platform for methodical risk assessments and quality management throughout the product lifecycle.
Why is Quality by Design So Important?
Quality by Design is important in pharmaceutical process development for several reasons:
Enhanced product quality and consistency
The QbD framework emphasizes understanding and controlling variability in manufacturing processes, ensuring that the final product consistently meets predefined quality criteria. This approach leads to more reliable and high-quality pharmaceutical products.
Regulatory compliance
Regulatory agencies like the FDA and EMA strongly support QbD principles. Applying the QbD framework facilitates regulatory submissions, as it demonstrates a thorough understanding of the process and product, potentially accelerating approval timelines and reducing the likelihood of regulatory hurdles.
Risk management
Quality by Design incorporates risk management strategies to identify, assess, and mitigate risks throughout the product lifecycle. By systematically evaluating potential risks and implementing control measures, companies can prevent issues before they arise, ensuring patient safety and product efficacy.
Efficiency and cost-effectiveness
Implementing QbD can lead to more efficient processes, reducing the need for extensive end-product testing and rework. This efficiency translates to cost savings in production and reduced time to market for new drugs.
Scientific understanding and process knowledge
The QbD approach requires a deep understanding of the product and process, including CQAs and critical process parameters (CPPs). This knowledge enables better decision-making, robust process design, and continuous improvement.
Robust control strategies
By identifying CQAs and CPPs, QbD helps in developing robust control strategies that ensure product quality. These strategies are based on scientific data and risk assessments, leading to more reliable and predictable manufacturing outcomes.
Flexibility and innovation
A focus on QbD promotes a flexible regulatory framework where changes in the manufacturing process can be managed through a well-understood design space. This flexibility encourages innovation and continuous improvement, allowing companies to adapt to new technologies and methodologies.
Knowledge management
Quality by Design emphasizes comprehensive documentation and knowledge sharing; this focus strengthens CMC data management, ensuring valuable information is not lost over time and can be reused in future projects, enhancing efficiency and fostering a culture of continuous improvement.
Patient safety
Ultimately, the primary goal of QbD is to ensure patient safety by delivering high-quality pharmaceutical products. By systematically designing quality into the manufacturing process, QbD minimizes the risk of product recalls and adverse effects, safeguarding public health.
What About Analytical Quality by Design?
Analytical Quality by Design (AQbD) is an extension of the QbD principles specifically applied to the development of analytical methods.
Just as QbD enhances pharmaceutical process development, AQbD brings significant advantages to the development and validation of analytical method, ensuring the methods are robust, reliable, and capable of consistently delivering accurate results.
Systematic method development
Analytical Quality by Design takes a structured approach to method development, starting with a clear understanding of the analytical target profile (ATP) and the critical method attributes (CMAs). This ensures the development process is thorough and systematic, leading to robust methods.
Improved method robustness and reliability
By identifying and controlling critical method parameters (CMPs) and their impact on method performance, AQbD enhances the robustness and reliability of analytical methods. This means the methods are less susceptible to variability and can consistently produce accurate and precise results.
Enhanced method understanding
Analytical Quality by Design involves a deep understanding of the analytical method and its performance characteristics. This knowledge helps scientists identify potential sources of variability and implement strategies to control them. It also aids in troubleshooting and continuous improvement of the method.
Regulatory compliance and flexibility
Regulatory agencies increasingly expect the application of QbD principles in analytical method development; AQbD facilitates smoother regulatory submissions by demonstrating a thorough understanding of the method and its robustness. Additionally, AQbD allows for greater regulatory flexibility, enabling method changes within the defined method operable design region (MODR) without the need for revalidation.
Lifecycle management
The AQbD framework supports the entire analytical method lifecycle, from development through validation to routine use. By continuously monitoring and improving the method based on performance data, AQbD ensures sustained method performance and reliability.
Data-driven decision-making
Analytical Quality by Design relies on statistical and risk-based approaches to make informed decisions during method development. This data-driven approach enhances the quality and reliability of the method, providing a solid foundation for making method-related decisions.
Facilitated knowledge transfer
The AQbD framework promotes comprehensive documentation and knowledge sharing. This facilitates the transfer of method knowledge within and between organizations, ensuring consistency and continuity in method application and improvement.
How ValGenesis iCMC™ Supports the Application of QbD Frameworks and CMC Lifecycle Management
ValGenesis iCMC is designed to seamlessly integrate QbD principles and risk management practices into the pharmaceutical development process, serving as a CMC lifecycle management platform for end-to-end oversight. Here's how:
- Early-Stage Development: Quality Target Product Profile (QTPP) and CQAs
At the outset, iCMC enables teams to define the Quality Target Product Profile (QTPP) and identify CQAs, providing the foundation for applying advanced pharma risk assessment tools throughout early-stage development. Each attribute is evaluated against various criteria, considering its impact and uncertainty. This systematic evaluation ensures that all critical aspects of the product are identified and prioritized from the beginning. - Process Mapping and Parameter Criticality
iCMC facilitates detailed process mapping, allowing teams to document every step and its associated process parameters. Correlating CQAs with specific process parameters helps establish a correlation matrix. This matrix assesses how each parameter impacts the quality attributes, aiding in the determination of their criticality. The rationale behind decisions is documented, promoting transparency and easy access to critical information. - Seamless Transition from Early-Stage to Late-Stage Development
As development progresses, iCMC ensures that risk assessments are seamlessly transferred from early to late stages. This continuity prevents information loss and rework, allowing teams to build upon existing knowledge. During late-stage development, pharma risk assessment tools like FMEA (Failure Modes and Effects Analysis) come into play, helping prioritize critical risks and develop mitigation strategies. - Automated Control Strategy and Reporting
iCMC's capabilities extend to the automated development of control strategies based on risk assessments. This automation accelerates the creation of robust control strategies essential for maintaining product quality. Real-time reporting features empower prompt collection and analysis of data, enhancing decision-making and regulatory compliance. - Knowledge Management and Reuse
One of the standout features of iCMC is its ability to facilitate knowledge management. Information from past projects, similar products, or different dosages can be reused with a single click, reducing the need to start assessments from scratch. This not only saves time but also promotes consistency and continuity across projects.
Why iCMC is Superior to Spreadsheets for QbD
Using a specialized CMC lifecycle management platform for QbD implementation in pharma process development offers numerous advantages over traditional spreadsheet-based methods.Pharmaceutical companies can ensure greater efficiency, improved data integrity, and better regulatory compliance, ultimately leading to higher quality and more reliable products.
ValGenesis iCMC features the following capabilities:
- Single Repository
The application provides a single repository for all data related to the QbD process. This ensures that relevant information is stored in one place, making it easily accessible and reducing the risk of data fragmentation. In contrast, spreadsheets are often scattered across disparate systems and physical locations, leading to potential data loss and inconsistencies. - Seamless Data Transition
As part of effective CMC data management, data moves easily from the early to late stages of development, maintaining continuity and ensuring that all stakeholders have access to the latest information. Spreadsheets require manual updates and transfers, increasing the risk of errors and data loss during transitions. - Smart Detection of Areas of Concern
Built-in intelligence automatically detects areas of concern, flagging potential issues based on predefined criteria and historical data. This enables proactive risk management. Spreadsheets lack this level of automation and require manual monitoring, which is time-consuming and prone to human error. - Collaborative Environment
iCMC facilitates a collaborative environment where multiple stakeholders can access and contribute to the same data in real time. This enhances communication and ensures that everyone is on the same page. Even when shared, spreadsheets do not offer the same level of real-time collaboration and version control. - One-Click Reporting
Robust reporting capabilities allow users to generate comprehensive reports with a single click. This includes real-time data visualization and automated report generation. Spreadsheets require manual data compilation and report creation, which is labor-intensive and inefficient. - Facilitated Knowledge Transfer
Knowledge and data from previous projects are preserved and easily transferable to new projects, supporting more efficient CMC data management across the lifecycle. This reduces redundancy and accelerates the development process. By contrast, spreadsheets often require manual consolidation of historical data, which can be cumbersome and incomplete. - Reusability of Assessments
There’s no need to start assessments from scratch—previous ones can be reused and adapted for new projects, saving time and resources. Spreadsheets do not inherently support this level of reusability and require manual effort to adapt existing data for new purposes. - Audit Trail and Regulatory Compliance
A comprehensive audit trail ensures compliance with regulations such as 21 CFR Part 11. All changes are tracked, and data integrity is maintained. Spreadsheets lack built-in audit trails and often do not meet regulatory requirements without extensive manual documentation and validation.
Top Four Benefits of iCMC in Pharmaceutical Process Development
- Enhanced Regulatory Compliance
By thoroughly understanding and documenting product and process risks, ValGenesis iCMC helps streamline regulatory submissions, reducing hurdles and expediting approval timelines.
- Accelerated Development Timelines
The application optimizes experimental design and risk assessment processes, leading to faster development of robust control strategies. - Improved Knowledge Sharing
Centralized storage of quality risk management information strengthens CMC data management, reducing knowledge loss and fostering a collaborative environment for diverse and dispersed teams. - Streamlined Knowledge Transfer
With iCMC, transitioning knowledge between stages or projects becomes effortless, ensuring continuity and efficiency.
From Framework to Practice
ValGenesis iCMC stands out as a comprehensive tool that integrates QbD principles and robust risk management into pharmaceutical and biopharmaceutical process development and analytical method development.
By providing a centralized, dynamic CMC lifecycle management platform for risk assessments and quality risk management, it enhances regulatory compliance, accelerates development timelines, and promotes effective knowledge sharing for companies committed to delivering high-quality products.
Read related content: Better Pharmaceutical Development: A Case for ValGenesis iCMC
Related Blog Posts

Electronic Logbooks: Leveraging the Power of Business Rules
ValGenesis e-Logbook uses business rules to enforce SOPs and standardize log activities, improving decision-making, productivity, and data integrity.
By Lisa Weeks
Read
FDAs Computer Software Assurance (CSA) – Part 3 of 3
The efficiencies gained, according to ValGenesis customers, range from 50% to 80% improvements. This lessens demand and allows more time to be focused on higher priority tasks. Knowing the ValGenesis VLMS ensures controls and standards are followed, according to customer-specific requirements, stakeholders are given a high-degree of assurance that Quality is ingrained in the process.
By Steve Thompson
Read
Developing a Drug Product Using QbD: a ValGenesis Story
This story is about our project with Libbs Farmacêutica to successfully apply a QbD framework in the development of an oral drug product
By Sofia Santos
Read