6 Ways to Make Your Risk Management More Effective


Effective risk management is crucial for life sciences manufacturers to ensure the safety, quality, and compliance of their products and patients. A robust risk management process not only safeguards against potential hazards but also enhances the overall quality of your operations. In contrast, a mere "check the box" approach to regulatory compliance can be counterproductive, diverting resources and hindering innovation in product development.
In this blog post, we'll look at the status quo of risk management and how to make your process more effective.
ICH Q9 (R1): The Guideline That Matters
The ICH Q9 guideline, which focuses on quality risk management (QRM) within the pharmaceutical industry, addresses the inherent risks associated with the manufacturing and utilization of drug products.
ICH Q9 underscores the critical importance of maintaining consistent product quality throughout the entire product lifecycle, ensuring that the attributes deemed essential for drug product quality during clinical studies remain unchanged as the product advances toward patient use.
An effective quality risk management approach, as advocated by ICH Q9, offers a proactive means of identifying and controlling potential quality issues during the development and manufacturing processes. It also enhances decision-making in response to quality-related problems.
This approach aims to benefit both the life sciences industry and regulatory authorities by providing a foundation for more informed and consistent risk-based decisions.
Additionally, it offers guidance on principles and tools for quality risk management, which can lead to better, more predictable outcomes for drug substances and drug products throughout their lifecycle.
What Should Your Quality Risk Management Process Look Like?
If you are following the ICH Q9 (R1) guideline, your quality risk management process should be a systematic process for the assessment, control, communication, and review of the risks to the quality of the drug (medicinal) product across the product lifecycle.
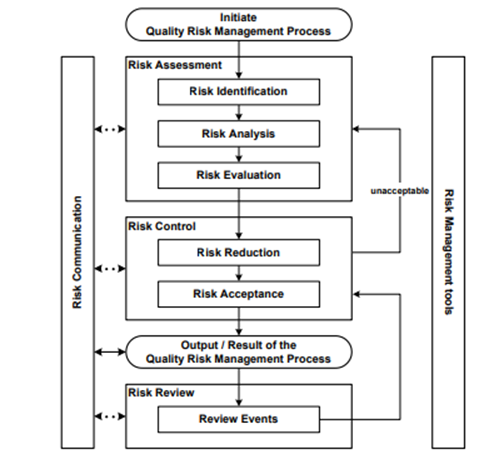
The process should involve interdisciplinary teams comprising experts from various areas, including the quality unit, business development, regulatory affairs, and more. Decision-makers are responsible for coordinating risk management across functions, defining the process, and ensuring adequate resources.
If we look at the process outlined in the ICH Q9 (R1) guideline, we have the following stages:
- Initiating a quality risk management process: Initiating the QRM process involves defining the problem or risk question, assembling relevant information and data, identifying a leader and necessary resources, specifying a timeline, deliverables, and the level of decision-making for risk management.
- Risk assessment: This stage includes the identification of hazards, analysis, and evaluation of risks associated with exposure to these hazards. It starts with a well-defined problem description or risk question, addressing what might go wrong, the likelihood of it happening, and the potential consequences.
- Risk control: Risk control focuses on accepting risks or reducing them to an acceptable level. Decision-makers use processes like benefit-cost analysis to determine the optimal level of risk control. It also considers the balance between benefits, risks, and resources.
- Risk reduction: Risk reduction aims to mitigate or avoid quality risks that exceed an acceptable level. It may involve actions to reduce the severity and probability of harm. However, it's essential to consider potential new risks introduced during this process.
- Risk acceptance: This stage involves making the decision to accept residual risk, either formally or passively, when residual risks are not explicitly specified. The acceptable level of risk depends on various parameters and should be determined on a case-by-case basis.
- Risk communication: Risk communication entails sharing information about risk and risk management between decision-makers and other stakeholders during any stage of the process. This not only ensures the proper documentation of information but also facilitates the sharing of knowledge among interested parties, regulators, industry, patients, and more.
- Risk review: Risk management is an ongoing part of quality management. The process should be continually reviewed and monitored for events (planned or unplanned) that might impact the original risk management decision, with review frequency based on risk level.
How to Make Your Risk Management More Effective
Here are six strategies you can implement to enhance the effectiveness of your risk management process, leading to improved safety, quality, and compliance outcomes.
- Abandon standalone documents: Integrated systems facilitate the exchange of risk data among different departments and stakeholders, fostering collaboration and guaranteeing that all parties are operating with the most current information. This might not be the case if you rely on standalone documents.
- Minimize the subjectivity of risk scoring: Inconsistencies in risk scoring throughout the product lifecycle can lead to unpredictable outcomes. Subjectivity plays a significant role in all aspects of the quality risk management process, particularly in the identification of hazards and the assessment of both the likelihood of occurrences and the severity of potential harm. It also influences the evaluation of risk reduction measures and the efficacy of the decisions that result from your quality risk management activities. While complete elimination of subjectivity may not be feasible, it can be mitigated by addressing bias and challenging assumptions.
At ValGenesis, we recommend (a) employing software that systematizes risk management activities. It is crucial that this software goes beyond mere systemization and also streamlines the risk-related processes across the organization. (b) Implementing a digital platform that has the capability to securely store and analyze data. This data can then be utilized as valuable feedback for the initial assumptions made during the initial risk management exercises. By enabling this virtuous circle, you can make QRM decisions that are both science- and knowledge-based, thus less subjective. - Optimize for efficiency: To boost efficiency, streamline your risk management process to ensure it does not consume excessive time and resources. This will enable quicker decision-making and promote progress without compromising quality. You can streamline by centralizing all your risk activities onto one platform. This unification will improve communication, reduce inefficiencies, and expedite the creation of more effective risk assessments. It will also eliminate the need to have all stakeholders connected simultaneously with asynchronous decision-making and save time and resources by minimizing the number of energy- and resource-draining meetings.
- Adopt continuous monitoring: Rather than depending on periodic reviews, implement automated systems that continuously monitor and gather pertinent data from multiple sources within the organization. This proactive approach allows for the creation of more comprehensive and informed risk assessments, leading to more accurate and expedited conclusions.
- Integrate Your QRM and QMS: To maximize effectiveness, do as much as you can to integrate your risk management with your quality management system (QMS). This integration of QRM and QMS yields numerous advantages at various stages and levels of the quality cycle. For instance, it can play a vital role in establishing quality objectives and policies and crafting and refining quality processes and procedures.
Furthermore, it can be instrumental in (a) validating and confirming the quality inputs and outputs, (b) overseeing and assessing performance metrics, (c) enhancing quality enhancement and change management efforts, and (d) delivering quality-focused training and awareness initiatives.
Combining QRM with other quality tools and methodologies fosters a robust quality culture, enhances customer satisfaction, and mitigates quality-related costs and risks, enhancing overall efficiency. - Leverage Lessons Learned: To continually improve effectiveness, you need to build on past experiences. Your QRM system should accumulate and utilize lessons learned from previous risk assessments, evaluations, controls, and post-market decisions. This accumulative knowledge empowers more informed risk management decisions over time.
How to Apply the 6 Strategies
First, implement a QRM System. Our preference is ValGenesis iRisk. ValGenesis iRisk is the world's most advanced risk management platform; it enables you to:
- Eliminate standalone documents by centralizing all the risk activities onto one platform.
- Optimize for efficiency by connecting and unifying teams with standard workflows and templates for greater insight and increased efficiency.
- Integrate your QRM with your QMS: iRisk enables seamless connectivity between most data sources and third-party applications.
- Leverage lessons learned as it serves as a valuable knowledge management tool, allowing you to use the acquired knowledge from previous risk assessments.
We also recommend ValGenesis Process Insight because it supports:
- Minimizing subjectivity in risk assessments.
- Continuous process monitoring.
Related Blog Posts

Risk Management of Sterilising Filtration
In this blog post we will talk about the perform Risk Management of Sterilising Filtration. We present the steps, the methods and the tools!
By Ricardo Leandro
Read
Medical Cannabis: the QRM Practices that Improve Your Business
A blog post where we give you some tips on how Quality Risk Management can help you improve your Medical Cannabis business
By Manuel Silva
Read
Digital Quality Risk Management: A ValGenesis Story
Learn about ValGenesis iRisk, an advanced tool for digital quality risk management, and its impact on vaccine development at a top biopharma company.
By Margarida Ventura
Read