Published on August 31, 2023
Reading time: -- minutes
Last updated on November 4, 2024


In a world dominated by rapid technological advancements, the pharmaceutical industry faces an ongoing struggle to adapt traditional risk assessment practices to the evolving complexity of operations and technologies.
One of the most formidable challenges in this endeavor is combating the inherent subjectivity deeply ingrained in the risk assessment processes. In this context, subjectivity pertains to the influence of personal biases and intuitions on risk evaluation. This can lead to inconsistent outcomes and decisions that may not fully prioritize patient safety and product quality.
Addressing subjectivity head-on and ushering in a data-driven approach is essential in a landscape of continuous improvement and heightened regulatory scrutiny.
Pharmaceutical product development risk assessment often falls prey to biased judgments influenced by preconceived notions, cultural factors, or individual preferences. Such biases are sourced from various personal experiences, cultural backgrounds, and professional affiliations. These biases cast a shadow over risk identification, analysis, and interpretation, distorting decision-making processes. Therefore, mitigating bias in risk assessment is pivotal for ensuring reliable outcomes.
We need transparent methodologies, diverse viewpoints, and independent reviews to overcome this issue. Only then will we minimize the impact of bias and promote objective risk assessment.
The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) has responded to the need for objective risk management by revising its guidelines.
The ICH Q9(R1) document now emphasizes formality in quality risk management (QRM), risk-based decision-making, minimizing subjectivity, and addressing product availability risks due to quality/manufacturing issues. These newly introduced sections pave the way for technology and data-driven approaches that mitigate the influence of bias and subjectivity in risk assessment.
Our strategy to tackle bias involves a digital framework for risk assessment and manufacturing insights. This framework taps into existing knowledge and evolves through iterative risk refinement. A digital QRM system equips organizations with templates, workflows, interconnected tools, and a knowledge repository, effectively reducing subjectivity. Meanwhile, a digital platform for manufacturing intelligence consolidates and interprets data from diverse sources.
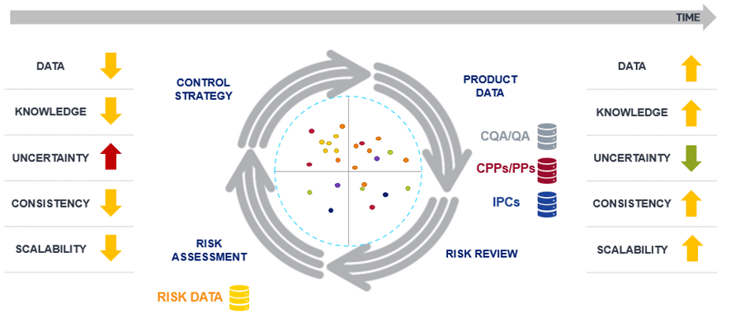
This approach yields significant benefits, especially in later stages of product development, such as Stage 3 (Continued Process Verification), per the FDA's Process Validation Guidance. Systematic data collection, trend analysis, and the detection of deviations become feasible sources of evidence for enhancing initial risk assumptions.
Adopting a data-driven risk assessment approach unlocks several advantages:
We explore further advantages in a recent blog post that delves into the huge benefits of integrating data and risk management.
In an environment where technological innovation often outpaces regulations, embracing QRM and ICH Q9(R1) through technology, data, and digital tools is essential.
Implementing a data-driven risk management approach sparks transformative change, enabling organizations to harness QRM's potential as a driver of continuous improvement, extending beyond regulatory compliance. This requires a holistic approach that merges risk and data, facilitated by digital platforms that offer a comprehensive view of a product's lifecycle. Such platforms provide a structured framework for generating, storing, analyzing, and managing knowledge over time.
As the pharmaceutical industry journeys toward a future shaped by innovation and precision, leveraging the power of data-driven risk assessment promises safer products, enhanced patient outcomes, and sustainable success.

Quality risk management software ensures compliance with ICH Q9(R1) guidelines, enhancing risk assessments, decision-making, and product safety in pharma.
By Margarida Ventura
Read
In this blog post we'll look into what steps you may need to take to make sure you are compliant with the ICH Q9(R1) guideline.
By Pedro Ferreira
Read.webp)
Discover how automated process monitoring in CPV ensures continuous control, early risk detection, and improved quality oversight in modern manufacturing.
By Sofia Santos
Read