Risk-based.
Justified Limits.
Digital Cleaning Validation.
ValGenesis iClean™ streamlines cleaning validation with global standardization, automated oversight, and scientific justification—ensuring audit readiness across all sites.
Impact Metrics
digital documentation and traceability with automated audit trails
faster cleaning validation cycle time through automation
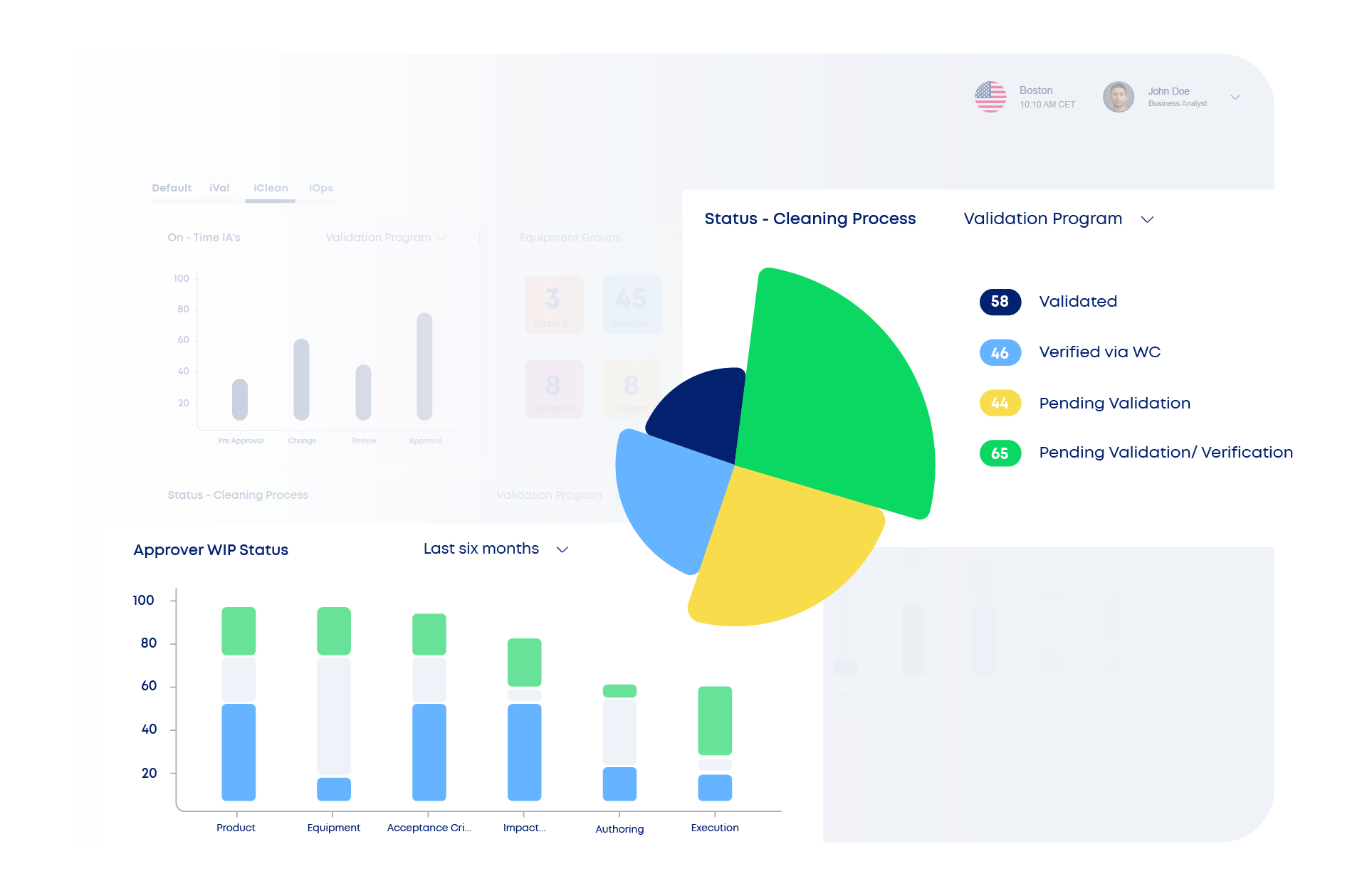
Discover How iClean Works as Part of Our Validation Lifecycle Suite
iClean digitalizes the entire cleaning validation lifecycle—from risk-based planning and MACO determination to execution and ongoing requalification. It automates calculations, integrates ADE/NOAEL-based scientific data, and uses 2D/3D equipment mapping to guide the sampling strategy.
With business-rule-driven decision trees, built-in residual limit enforcement, and a centralized method and agent repository, iClean helps teams reduce manual work, eliminate errors, and maintain global consistency. Its change control and requalification workflows ensure sustained compliance—even as processes evolve.
Overview
Accelerate compliance, reduce risk
Automate MACO calculations, validation cycles, and documentation for faster, inspection-ready delivery.
Standardize globally, flex locally
Centralize cleaning strategies while adapting validation protocols to site-specific and equipment-specific requirements.
Gain real-time oversight
Visualize status, risks, and readiness across sites through built-in analytics and intelligent dashboards.
Resources

The Future of Cleaning Validation
Modernize cleaning validation with digital CPV, better documentation, and ...

Unlocking Operational Capacity with Fully Digitalized Cleaning Validation
Cut cleaning-validation downtime and audit stress with a fully digital ...
Frequently Asked Questions
Do you need help with something or have questions about any features?
Ready for Smarter Cleaning Validation?
Simplify cleaning validation. Ensure audit readiness. Get started with ValGenesis iClean™.