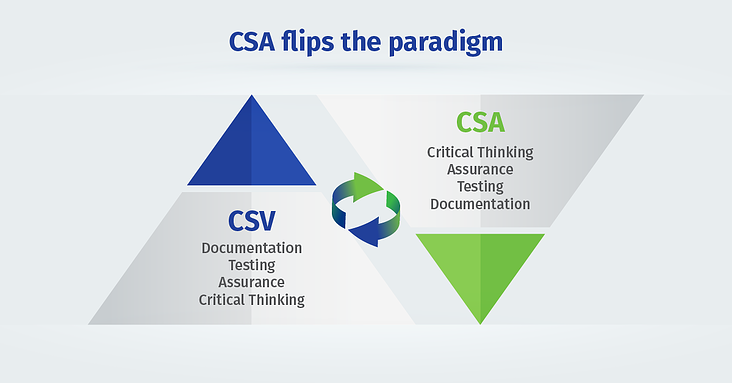
The draft guidance on computer software assurance (CSA) is a complete paradigm shift from document-focused computer system validation to critical thinking assurance practices.
This isn't nearly as dramatic as it sounds.
The FDA has advised life sciences companies to take a risk-based, least burdensome approach to CSV for two decades. These guidelines are not new. Adhering to them, however, has been a challenge for many organizations. Enter CSA.
What is CSA in a Nutshell?
The CSA methodology supports product quality and patient safety by emphasizing the use of critical thinking and digital technologies over burdensome testing and documentation. By streamlining the validation process, CSA can help you achieve faster deployment and ROI.
CSV is not going away. Riskier applications will always require rigorous validation. But CSA is the wave of future and a better way to perform CSV.
Articles and Podcasts About CSA
Check out our 5-part series devoted to CSA. Written by validation expert Steve Thompson, each post includes a link to a companion podcast that offers a deeper dive into the topic.
Post #1 Computer Software Assurance: What's All the Hype?
CSV and CSA aren't all that different. So then, what's all the hype about? This post defines CSA and explains why (and when) the FDA encourages you to flip the CSV script.

Post #2 Critical Thinking and Its Role in Computer Software Assurance
Critical thinking is a vital life skill and the principal requirement of CSA. This post teaches you how to hone your critical thinking skills and how to apply those skills to CSA.
Post #3 Computer Software Assurance: What are Assurance Needs?
In the new CSA methodology, assurance needs are at the top of the paradigm, second only to critical thinking. This post explains how to identify the assurance needs of the system and the activities you must perform to address those needs.
Post #4 How Computer Software Assurance Will Impact Traditional CSV Testing
CSA encourages the use of unscripted test methods, such as ad hoc testing, and automated technologies. This post reveals the new testing methods at your disposal and the root cause of most testing issues.

The thinking is that the more paper you generate, the better you validate. According to the FDA, this is not only wrong, it's dangerous. This post explains why.