Published on March 22, 2023
Reading time: -- minutes
Last updated on November 4, 2024


The last few years have been full of advances in the field Advanced Therapy Medicinal Products (ATMPs). And, as any products that interfere in the health and well-being of patients, it requires a Quality Risk Management System. Which brings us to this blog post: why a Digital QRM System for ATMPs is a must have!
We mentioned advances in the field of ATMPs. These advances have the shape of cellular and molecular biotechnology progress that affects gene therapy, somatic cell therapy and tissue engineering.
Across the world, the ICH Q9 is the standard for Quality Risk Management, including ATMPs.
But if we look at the United States in specific, the FDA has issued the Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs).
This guidance focuses on the following points:
In Europe, on the other hand, EMA issued this regulation on advanced therapy medicinal products (known as ATMPs Regulation!).
This ATMPs Regulation includes specific guidance for ATMPs for:
And this is where we want to focus: the Risk Management of ATMPs!
As with other medical products, risk detection should start as early as possible and continue throughout the development. This allows you to minimize and prevent risks when it’s possible!
Thus, the safety and efficacy are managed through the risk management plan, as the figure shows:
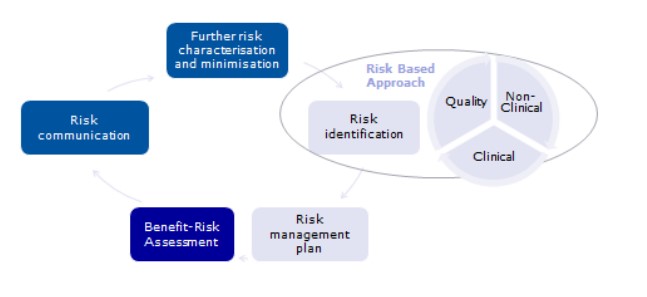
The same applies for ATMPs.
However, the scope of the risks is a bit different than traditional pharma and biopharma. Let’s see a few examples:
Risk identification is then followed by the construction of the Risk Management Plan, the benefit-risk assessment identification and then taking risk minimization measures. And then you need to measure the effectiveness of these Risk minimization measures.
As you know, for each ATMP product you have, you will need to establish your Quality Target Product Profile (QTPP). Then, from there, you can build your list of Critical Quality Attributes (CQA) and Critical Process Parameters (CPPs).
As a result of that, and by keeping a level of measurement and control over these CQAs and CPPs, you can make sure the ATMPs you produce have the desired quality.
Then you may ask: what is the most important aspect of the QRM process? We believe that the key point is to have a standard application of risk management procedures. The reasons are simple: simplification, replicability, data and information integrity and the possibility to use information through several processes.
Obviously, the challenge here is having that standard application of risk management principles. Which is where having a Digital QRM System for ATMPs comes in.
If you have a digital QRM platform where all the information is centralized and shared, it can bring you a whole suit of benefits:
If we haven’t convinced you by now, let’s state the obvious once again: Digitalization will support your decision-making providing you an understanding of the risks each choice brings.
ValGenesis has a lot of experience digitalizing QRM Processes for ATMPs – including Cell & Gene Therapy processes.
If you think that your team would benefit from the process that we have just described, we welcome you to check out our service and reach out to us. We can discuss what could be the better options to make sure your processes lose the least – and therefore gain the most!

The new ICH Q13 guideline is almost here! ValGenesis' experts have been actively involved on the public consultation phase and, we have something to say about it.
By Rui Silva
Read
Paper-based workflows have been the industry standard for validating systems and processes. But companies gain many benefits from going digital. (Part 2 of 2)
By Lisa Weeks
Read
There's a need to accelerate pharma product development and process scale-up. Having an effective tech transfer strategy can help you.
By Sofia Santos
Read