How ValGenesis VLMS fosters a Quality-Focused Culture
This is the third-and-final write-up on FDA’s Computer Software Assurance (CSA) explains how the ValGenesis Validation Lifecycle Management System (VLMS) fosters a quality-focused culture.
Here are the Prior Blogs for Your Convenience
- How to accelerate adoption of FDA’s Computer Software Assurance
- Changing Compliance-Centric Mindsets
How does the ValGenesis’ VLMS foster a quality-focused culture? First, rather than forcing people to comply or else, the ValGenesis VLMS implements technical controls and functionality that consistently performs as intended, eliminating subjectivity and delivering to quality standards.
Always on the Right Path
Second, ValGenesis customers have repurposed some Standard Operating Procedures (SOP) through implementation of the technology controls available in the VLMS, mentioned above. The benefit the assurance that procedure is followed; it must be because the only valid path forward is the one designed by Subject Matter Experts (SMEs), approved by Quality Assurance (QA), and implemented in the VLMS.
Assessments Right From the Start
Third, configured and approved assessments guide authorized users through decision trees, tailored to customer specific requirements, and result in assignment of a Framework that ensures all required validation deliverables will be completed before a validated state can be achieved. This starts right at the beginning of the validation lifecycle. Implementing risk assessments through technology removes subjectivity, delivers consistence and improves quality.
Quality Awareness From the Start
Forth, QA is aware, has agreed and approved the process (technical controls) at the onset. Validation proves the controls are effective, are adhered to, and tracked through a comprehensive audit trail that’s readily verifiable. Consistency and quality are attained through with assurance that only approved, current, and effective templates are used. When QA receives notification to review-and-approve, they have a high degree of assurance the majority of the validation package is in compliance. Quality-by-Review expedites the QA approval process.
Everybody’s Job Becomes Easier
The efficiencies gained, according to ValGenesis customers, range from 50% to 80% improvements. This lessens demand and allows more time to be focused on higher priority tasks. Knowing the ValGenesis VLMS ensures controls and standards are followed, according to customer-specific requirements, stakeholders are given a high-degree of assurance that Quality is ingrained in the process. As burdens lessen, and issues are averted, the benefit of investing in Quality, right from the start, becomes obvious. Culture morphs from a compliance-centric mindset that’s highly subjective, to a Quality-focused culture that’s objective, compliant, and defendable.
Aligned With CSA
The ValGenesis VLMS is not only aligned with FDA’s Computer Software Assurance (CSA) initiative, the it also expedites an organizations ability to adopt and deploy CSA..
Figure 1 illustrates the CSA approach. In this three-part blog series, we’ve walked through each phase and highlighted how the ValGenesis VLMS helps companies achieve results.
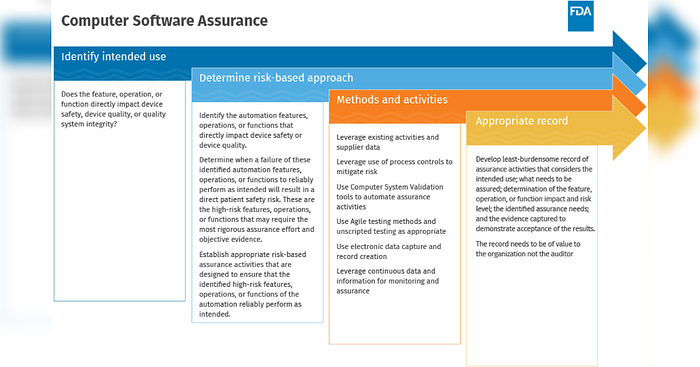
Figure 1 - How to CSA1(SOURCE: Cisco Vincenty, FDA Case for Quality Program Manager (FDA))
If you would like more information, please don’t hesitate to contact us.
Resources



